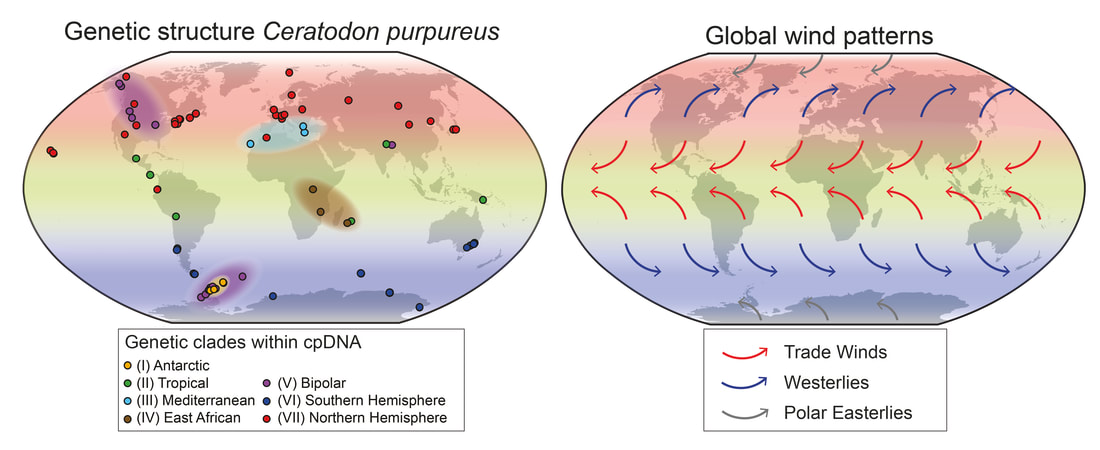
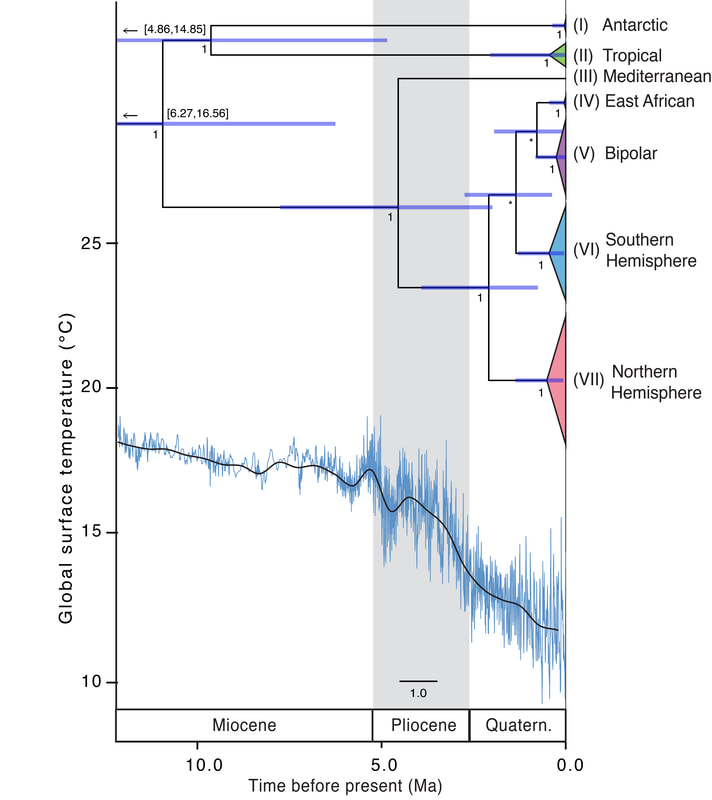
Old Lineages in Distinct Biogeographic Regions Additionally, the rise of modern flammable grass-, shrub- and woodlands (late Miocene onwards with peak origins in late Pliocene; Bond, 2015) could have promoted the spread of C. purpureus, as the species is also frequently found in fire-influenced habitats (it is even called “Fire Moss” as a common name in English). Furthermore, in the recent post-quaternary period, the origin and expansion of urban environments provided major sources of anthropogenically influenced disturbance potentially favorable to C. purpureus. Multiple Antarctic Colonizations, Including an Ancient Lineage We found at least three dispersal events to the Antarctic (see clades I, V and VI above). This reveals that Antarctica is not as isolated as is often assumed for spore-dispersed organisms (e.g. also seen in the Antarctic moss Chorisodontium aciphyllum; Biersma et al., 2018a). However, the analyses also revealed one old Antarctic clade (I), possibly isolated on the continent since the late Miocene or early Pliocene. Although more extensive sampling may be required to fully assess whether clade I is limited to Antarctica, its apparent ancient isolation suggests it may be a remnant lineage that has survived past glaciations in the maritime Antarctic in situ. Molecular, phylogenetic and biogeographic studies also suggest in situ survival for many groups of terrestrial fauna in Antarctica throughout the Quaternary, Neogene and even Paleogene (see Convey et al., 2008, 2009, 2020, and references therein). Recently, increased evidence has also been found of million-year persistence of the Antarctic flora, e.g. several endemic species of Schistidium (Biersma et al., 2018b), and Bryum argenteum (Pisa et al., 2014). Here, our data indicate that at least one lineage (I) of C. purpureus may also have had a long-term Antarctic presence in situ. Relevance for other organisms and evolutionary studies Our general findings may also be relevant to understanding global environmental influences on the biogeography of other organisms with microscopic propagules (e.g., spores) dispersed by wind. The findings may also be of relevance to further evolutionary studies on bryophytes, as C. purpureus is commonly used as a model organism in genetic, physiological, and developmental studies, in particular for studying the evolution of developmental processes in bryophytes (e.g. McDaniel et al., 2007, and references therein; Szövényi et al., 2014). For this type of developmental research, good baseline knowledge on the evolutionary history and global biogeography of a species is fundamental, for instance, for underpinning interpretation of crossing experiments, trait mapping and marker discovery, and controlling for demographic or population effects. The matrilineal biogeographic structure identified here therefore provides a useful framework for future genetic and developmental studies on bryophytes. For more information see: Biersma, E.M., Convey, P., Wyber, R., Robinson, S.A., Dowton, M., Van De Vijver, B., Linse, K., Griffiths H. & Jackson, J.A. (2020) Latitudinal biogeographic structuring in the globally distributed moss Ceratodon purpureus. Front. Plant Sci. 11:502359. https://doi.org/10.3389/fpls.2020.502359 References: Biersma, E. M., Jackson, J. A., Bracegirdle, T. J., Griffiths, H., Linse, K., Convey, P. (2018a). Low genetic variation between South American and Antarctic populations of the bank−forming moss Chorisodontium aciphyllum (Dicranaceae). Polar. Biol. 41, 599–610. doi: 10.1007/s00300-017-2221-1 Biersma, E. M., Jackson, J. A., Stech, M., Griffiths, H., Linse, K., Convey, P. (2018b). Molecular data suggest long-term in situ Antarctic persistence within Antarctica’s most speciose plant genus, Schistidium. Front. Ecol. Evol. 6:77. doi: 10.3389/fevo.2018.00077 Bond, W. J. (2015). Fires in the Cenozoic: a late flowering of flammable ecosystems. Front. Plant Sci. 5:749. doi: 10.3389/fpls.2014.00749 Convey, P., Gibson, J. A., Hillenbrand, C. D., Hodgson, D. A., Pugh, P. J., Smellie, J. L., et al. (2008). Antarctic terrestrial life - challenging the history of the frozen continent? Biol. Rev. Camb. Philos. Soc 83, 103–117. doi: 10.1111/j.1469-185X.2008.00034.x Convey, P., Bindschadler, R., Di Prisco, G., Fahrbach, E., Gutt, J., Hodgson, D. A., et al. (2009). Antarctic climate change and the environment. Antarct. Sci. 21, 541–563. doi: 10.1017/S0954102009990642 Convey, P., Biersma, E. M., Casanova-Katny, A., Maturana, C. S. (2020). “Refuges of Antarctic diversity,” in Past Antarctica. Eds. Oliva, M., Ruiz-Fernández, J. (Cambridge, MA: Academic Press), 181–200. doi: 10.1016/B978-0-12-817925-3.00010-0 Hansen, J., Sato, M., Russell, G., Kharecha, P. (2013). Climate sensitivity, sea level and atmospheric CO2. Philos. Trans. R. Soc A. 371, 20120294. doi: 10.1098/rsta.2012.0294 Kops, J., Hartsen, F. A., van Eeden, F. W. (1868). Flora Batava, of Afbeeldingen en Beschrijving van Nederlandsche gewassen. XIII Deel 13(Amsterdam, the Netherlands: J. C. Sepp en Zoon). McDaniel, S. F., Willis, J. H., Shaw, A. J. (2007). A linkage map reveals a complex basis for segregation distortion in an interpopulation cross in the moss Ceratodon purpureus. Genetics 176, 2489–2500. doi: 10.1534/genetics.107.075424 Pisa, S., Biersma, E. M., Convey, P., Patiño, J., Vanderpoorten, A., Werner, O., et al. (2014). The cosmopolitan moss Bryum argenteumin Antarctica: recent colonisation or in situ survival? Polar. Biol. 37, 1469–1477. doi: 10.1007/s00300-014-1537-3 Szövényi, P., Perroud, P. F., Symeonidi, A., Stevenson, S., Quatrano, R. S., Rensing, S. A., et al. (2014). De novo assembly and comparative analysis of the Ceratodon purpureus transcriptome. Mol. Ecol. Resour. 15, 203–215. doi: 10.1111/1755-0998.12284
2 Comments
|
Hi! I am Elise Biersma, an evolutionary biologist studying polar plants and microbes.Archives
January 2021
Categories |



 RSS Feed
RSS Feed